Answers
Answer:
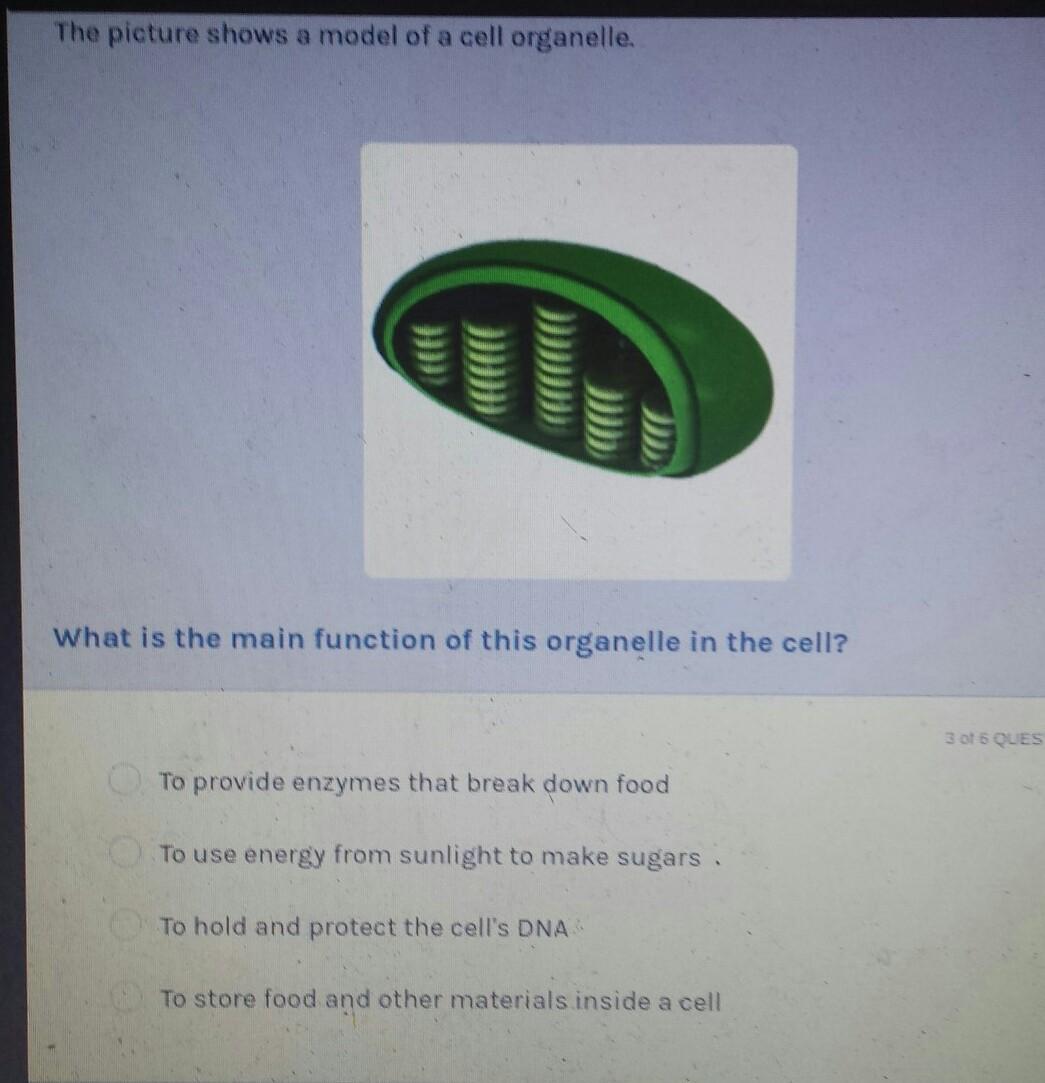
[tex]to \: use \: energy \: from \\ \: sunlight \: to \: make \: sugars.[/tex]
Explanation:
[tex]the \: main \: function \: of \: this \: organelle \\ \: in \: the \: cell \: is \: to \: use \: energy \: from \\ \: sunlight \: to \: make \: sugars.[/tex]
Related Questions
Two people are pulling on a rope, like in the picture below. The person on the left pulls at 5 N and the person on the right pulls 5 N. What is the net force on the rope?
Answers
Answer:
The net force is 0
Explanation:
because both people on either side are pulling the rope with the same force
4. Describe the arrangement of the electrons on each energy level.
Answers
Answer:
Explanation:
If the energy of an atom is increased, an electron in the atom gets excited. To go back to its ground state, the electron releases energy. The energy of the light released when an electron drops in energy level is the same as the difference in energy between the two levels.
Viewed simply, electrons are arranged in shells around an atom’s nucleus. Electrons closest to the nucleus will have the lowest energy. Electrons further away from the nucleus will have higher energy. An atom’s electron shell can accommodate 2n2 electrons (where n is the shell level).
In a more realistic model, electrons move in atomic orbitals, or subshells. There are four different orbital shapes: s, p, d, and f. Within each shell, the s subshell is at a lower energy than the p. An orbital diagram is used to determine an atom’s electron configuration.
There are guidelines for determining the electron configuration of an atom. An electron will move to the orbital with lowest energy. Each orbital can hold only one electron pair. Electrons will separate as much as possible within a shell.
In an atom, electrons are arranged in shells that surround the nucleus, with each successive shell moving further away from the nucleus.
What is energy level?In physics, an energy level is any discrete value from a set of total energy values for a subatomic particle confined by a force to a limited space or for a system of such particles, such as an atom or a nucleus.
Energy levels are fixed distances from an atom's nucleus where electrons can be found. Electrons are tiny, negatively charged particles that move around an atom's positive nucleus. The steps of a staircase represent energy levels.
Around the nucleus, electrons are arranged in different shells. Each successive shell can only hold so many electrons. First, the innermost shell is filled. This shell can only contain two electrons.
Thus, this way, the electrons are arranged.
For more details regarding energy level, visit:
https://brainly.com/question/17396431
#SPJ6
when Lithium forms an ionic compound with carbonate, how many of each ion is needed?
Answers
Answer:
Two Lithium ions and one carbonate ion
Explanation:
Let us look at this equation;
Li2CO3(s) ------->2Li^(aq) + CO3^2-(aq)
We can see that each unit of Li2CO3 has two lithium ions and one carbonate ion.
Let us not forget that Li has a valency of 1 while carbonate ion has a valency of 2. Exchange of valencies gives the final formula of the compound.
Answer:
Two Lithium ions and one carbonate ion
Explanation:
Which of the following is used for chemical symbols today? a. drawings c. letters b. icons d. numbers
Answers
Answer:
Letters
Explanation:
For example, today we use the periodic table which is full of elements named with 1 or 2 letters. Like how Helium is He and Sodium is Na. Hope this helps!!!
Chemical symbols are used to identify an element and it is represented by one or two letters from their latin name. Thus option c is correct.
What is chemical symbol?Chemical symbols are representations for elements and compounds. Each element in the periodic table have their own symbol which first letter or first two letters from their latin name.
For example, hydrogen has the symbol H, oxygen is O, N for nitrogen C for carbon, Na for sodium etc. The latin name of sodium is natrium, that's why it has the symbol Na.
The letters used in the chemical symbol must be capital. If two letters are there like in Na, Ar etc. the first letter be capital and second letter have to be in small case.
In a similar way compounds can be represented using chemical symbols with number of each atom given as subscripts of the elemental symbol as in CO₂, H₂O etc.
To find more on chemical symbol, refer here:
https://brainly.com/question/9249660
#SPJ6
An unknown substance has a volume of 2 cm3 and a mass of 38.6 grams.
What is the density of the sample?
Answers
Answer:
The answer is 19.3 g/cm³Explanation:
The density of a substance can be found by using the formula
[tex]density = \frac{mass}{volume} \\ [/tex]
From the question we have
[tex]density = \frac{38.6}{2} \\ [/tex]
We have the final answer as
19.3 g/cm³Hope this helps you
Identify the mineral that can have the composition of KMg3AlSi3O10(OH)2
Answers
Answer:
Phlogopite
Explanation:
It is a member of mica group family of phyllosilicates mineral. It is the magnesium endmember of the biotite stable - the chemical formulation KMg3AlSi3O10 (F, OH) 2.
What is the mass, in grams, of 0.450 moles of Sb?
Answers
Answer:
54.9 g
Explanation:
0.450 mol x 122g/mol
Relative and average atomic mass both describe properties of an element related to its different isotopes. Out of these two Relative atomic mas is more accurate. Therefore, 54.9 g is the mass in grams of 0.450 moles of Sb.
What is mass?Mass defines the quantity of a substance. It is measured in gram or kilogram. Average mass is the mass of atoms of an element that are isotopes. It can be calculated by multiplying mass of a isotope to natural abundance of that isotope.
Average atomic mass = (mass of first isotope× percent abundance of first isotope)+(mass of second isotope× percent abundance of second isotope)
Mass of Sb= number of moles of Sb ×Molar mass of Sb
=0.450 mol x 122g/mol
=54.9 g
Therefore, 54.9 g is the mass in grams of 0.450 moles of Sb.
To learn more about mass, here:
https://brainly.com/question/28704035
#SPJ6
A
Carpet is beaten to
beaten to remove dust
Answers
Answer:
It's Clean?
Explanation:
3. Which of the following is not a compound?
water
potassium iodide
B
sodium chloride
D hydrogen
Answers
Which phrase best describes igneous rocks?
form when lava and magma cool
accumulate sediments following erosion
require heat and pressure to be formed
consist of many sediment layers
ctivity
Answers
Answer:
A) form when lava and magma cool
please give me brainliest :p
God bless!
Answer:
A: form when lava and magma cools
Explanation:
Got it right on Edge 2020 :)
Determine the amount of energy (heat) in Joules and Kcal required to raise the temperature 0f 98.5 grams water from 37.0 0C to 75.0 0C.
Answers
Answer:
15660.712J
Explanation:
Q = m × c × del T
Q(energy) = ?
m (mass) = 98.5g
c = 4.184J
del T = 75-37 = 38 °C
Q = 98.5 × 4.184 × 38
Q = 15660.712 J
Which further observation led Mendeleev to create the periodic table
Answers
Answer:
Mendeleev realized that the physical and chemical properties of elements were related to their atomic mass in a 'periodic' way, and arranged them so that groups of elements with similar properties fell into vertical columns in his table.
Explanation:
(100 POINTS) When thermal energy (heat) is added to a reaction, it happens _________ (faster or slower)
When thermal energy (heat) is removed from a reaction, it happens _________ (faster or slower)
Answers
Answer:
1) faster
2) slower
Explanation:
Answer:
faster
Explanation:
You check the air pressure in your car tires on Monday morning and the pressure
reads 765 mmHg. A cold front drops the temperature considerably overnight. You
check your tire pressure Tuesday morning and the pressure reads 740 mmHg.
Using what you've learned about the properties of gases, what causes this change
in pressure?
Answers
The quantum mechanical model...
a
describes electrons as moving through specific circular orbits, and not staying in one place.
b
places electrons in fixed positions, not moving through specific orbits or orbitals.
c
describes electrons as moving anywhere in the atom, not specific circular orbits.
d
describes electrons as moving through cloud-like orbitals, not specific circular orbits.
Answers
Can you help me please
Answers
what are your steps to this from the lesson
A dull metal object has a density of 8.8 G/ML and a volume of 20 ML calculate the mass
Answers
Answer:
Mass = 0.000176 gram
Steps:
m = V × ρ
= 20 milliliter × 8.8 gram/cubic meter
= 2.0E-5 cubic meter × 8.8 gram/cubic meter
= 0.000176 gram
Explanation:
Write a complete, balanced chemical equation where tin metal reacts with aqueous hydrochloric acid to produce tin(II) chloride and hydrogen gas. Include states.
From the equation, which element is oxidized, and which element is reduced?
Answers
Answer:
1. The balanced equation is given below:
Sn (s) + 2HCl (aq) –> SnCl₂ (aq) + H₂ (g)
2a. H is oxidized.
2b. Sn is reduced.
Explanation:
1. Balanced equation for the reaction between tin (Sn) metal and aqueous hydrochloric acid (HCl) to produce tin(II) chloride (SnCl₂) and hydrogen gas (H₂).
This is illustrated below:
Sn (s) + HCl (aq) –> SnCl₂ (aq) + H₂ (g)
There are 2 atoms of Cl on the right side and 1 atom on the left side. It can be balance by putting 2 in front of HCl as shown below:
Sn (s) + 2HCl (aq) –> SnCl₂ (aq) + H₂ (g)
Now, the equation is balanced
2. Determination of the element that is oxidize and reduced.
This can be obtained as follow:
We shall determine the change in oxidation number of each element.
NOTE:
a. The oxidation number of H is always +1 except in hydrides where it is –1.
b. The oxidation state of Cl is always –1.
Sn (s) + 2HCl (aq) –> SnCl₂ (aq) + H₂ (g)
For Tin (Sn):
Sn = 0
SnCl₂ = 0
Sn + 2Cl = 0
Cl = – 1
Sn + 2(–1) = 0
Sn – 2 = 0
Collect like terms
Sn = 0 + 2
Sn = +2
Therefore, the oxidation number of Tin (Sn) changes from 0 to +2
For H:
H = +1
H₂ = 0
The oxidation number of H changes from +1 to 0
For Cl:
Cl is always –1. Therefore no change.
Summary:
Element >>Change in oxidation number
Sn >>>>>>>From 0 to +2
H >>>>>>>>From +1 to 0
Cl >>>>>>>No change
Therefore,
Sn is reduced since its oxidation number increased from 0 to +2.
H is oxidized since it oxidation number reduced from +1 to 0
The balanced chemical equation for the reaction is
Sn(s) + 2HCl(aq) → SnCl₂(aq) + H₂(g)
and the element that oxidized is tin (Sn) while the element that is reduced is hydrogen (H)
The balanced chemical equation for the reaction where tin metal reacts with aqueous hydrochloric acid to produce tin(II) chloride and hydrogen gas is
Sn(s) + 2HCl(aq) → SnCl₂(aq) + H₂(g)
From the equation,
The element that is oxidized is tin (Sn), because the oxidation state of Sn increased from 0 to +2
The element that is reduced is hydrogen (H), because the oxidation state of H decreased from +1 to 0.
Hence,
The balanced chemical equation for the reaction is
Sn(s) + 2HCl(aq) → SnCl₂(aq) + H₂(g)
and the element that oxidized is tin (Sn) while the element that is reduced is hydrogen (H).
Learn more here: https://brainly.com/question/12913997
In which situation is it better to have low friction?
Choose the correct answer.
a skydiver uses a parachute
a student walks down a hallway
a ladder leans against the wall
a hockey puck slides toward the goal
Answers
Answer: D- a hockey puck slides toward the goal
Explanation:
It needs to be able to slide easily and thats the correct answer on the test
Identify whether each element is a halogen, a noble gas, or nonmetal only.
Astatine (At):
Nitrogen (N):
Krypton (Kr):
Chlorine (Cl):
Sulfur (S):
Answers
Answer:
Astatine: Halogen
Nitrogen: Non-Metal
Krypton: Non-Metal, Noble Gas
Chlorine: Non-Metal
Sulfur: Non-metal
Explanation:
what is 1.23 x 10^-3 in standard notation
Answers
Answer:
=0.00123
Explanation:
Look at the attachments below
Hope this helps (:
Answer:
0.00123
Explanation:
Standard notation is the normal way of writing numbers. Examples include 1, 2, and 10. The number 1.23 x 10^-3 is written in scientific notation. The decimal goes after the first nonzero integer and it is multiplied by a power of 10. The power or exponent attached to the 10 tells you how many places over you need to move the decimal to get back into scientific notation. Examples include 1.00 x 10^2 (representing 100 in standard form because you would move the decimal two places to the right.), 2.0 x 10^1 (representing 20 in standard form because you would move the decimal one place to the right), and 3.0 x 10^-4 (representing 0.0003 in standard form because you would move the decimal four places to the left since it is a negative exponent).
The negative (-3) exponent in 1.23 x 10^-3 indicated to move the decimal three places to the left. If it was positive, you would move it three places to the right.
In 1.23 x 10^-3 move the decimal to the left 1 place to get:
0.123
two places to get:
0.0123
and a third place to get:
0.00123
The final answer is 0.00123
How many moles are in 12.6 grams of K2S?
Answers
The bright red line from the emission spectrum of hydrogen has a wavelength of 657 nm. What is the energy, in joules, of a single photon of this light? 4.35 x 10–40 J 3.03 x 10–28 J 3.03 x 10–19 J 4.56 x 1014 J
Answers
Answer:
3.03 × 10^-19 J
Explanation:
The energy, E, of a photon of light can be calculated by using the formula:
E = hv
Where; E is energy in Joules,
h is Planck's constant {6.626×10^−34J}
v is frequency of light
For this question, the wavelength (λ) is given, not the frequency (v).
Hence, we use;
v= c/λ
Where v= frequency, c= speed of light (3×10^8m/s), λ= wavelength (657nm)
657nm = 657 × 10^-9m
v = 3×10^8/657 × 10^-9
v = 3/657 × 10^(8--9)
v = 0.00456 × 10^17
v = 4.56 × 10^14
Since frequency (v) = 4.56 × 10^14;
E = hv
E = 6.626×10^−34 × 4.56 × 10^14
E = 30.21 × 10^(-34+14)
E = 30.21 × 10^-20
E = 3.021 × 10^-19
Therefore, the energy of the photon of light is 3.02 × 10^-19 J
whats the lewis dot diagram for calcium
Answers
Draw the Lewis dot structure for each atom of the compound to show how many valence electrons are present in each atom. For example, the calcium atom in calcium chloride, CaCl2, has two valence electrons, and the chlorine atoms have seven valence electrons each.
Who still uppppppp and boreddddd
Answers
Answer:
me
Explanation:
during recycling, ground glass is melted under light heat and poured into molds. which type of change occurs as the glass melts?
Answers
Hope this helps!
The state change occurs when the glass melts.
What are different states of matter?The matter is anything that has mass and occupies space.
The different states of matter are solid, liquid, gas, and plasma.
The properties of the different types of matter can be understood by looking at the arrangement of molecules.
When a solid substance is heated beyond its melting point, it changes its state from solid to liquid.
When liquid is heated beyond its vaporizing point, it changes its state from liquid to gas.
Solids are closely packed and have a definite shape and size.
Liquids are loosely packed and take up the space of the container.
Gas has high intermolecular spacing and the gases are not rigid.
Ground glass is melted under light heat and poured into molds.
Recycling glass involves changing the state of glass from solid to liquid state.
To know more about Different states of matter
https://brainly.com/question/1134312
#SPJ6
Question 2 of 10
What are Van der Waals forces?
A. Forces that change polar molecules into nonpolar molecules
B. Very strong forces that exist between two different molecules
O c. Hydrogen bonds in nonpolar molecules caused by a permanent
dipole
D. Small dipole attractions between molecules caused by a
temporary electron shift
SEB
Answers
Answer:
D
Explanation:
Van der Waal forces are small dipole attractions between molecules caused by a temporary electron shift ,here, the option D is correct.
What are Van Der Waal forces?Van der Waals forces exist among all kinds of atoms and molecules. The origin of this force stems from the instantaneous dipole-induced dipole interactions among adjacent polar atoms and molecules.
Among polar molecules, there are three components that contribute to the total forces: the induction force, the orientation force and the dispersion force.
The Van Der Waal forces that act between macroscopic bodies and surfaces in a solvent medium are relevant to the phenomena of protein adsorption.
Van Der Waal forces are the small dipole attractions between molecules caused by a temporary electron shift , thus, option D is correct.
Learn more about Van Der Waal forces here:
https://brainly.com/question/14395915
#SPJ2
identify the elements given the orbital diagrams listed below.
Answers
Answer:
See explanation.
Explanation:
1. There are 8 electrons. Elements that end with 2p orbitals are in the 2nd period (aka row) of the periodic table. Elements that have 4 electrons in 2p are in the 16th group (aka column) (column 16 may also be referred to as 6A) of the periodic table. So looking at row 2, column 16, we can see that the first diagram is of O, Oxygen.
2. 8 electrons. This is the same diagram as the one above.
3. 13 electrons. Elements ending with 3p are in period 3. Elements with 1 valence electron in a p orbital are in group 13 (aka group 3A).
4. 7 electrons. We already know 2p is period 2. 3 valence electrons in a p orbital means that it is in group 15/group 5A.
I did not write the answers for #3 and 4 but they can be easily found on a periodic table with the info I gave.
The elements given the above orbital diagrams are as follows:
Oxygen
Oxygen
Aluminum
Nitrogen
In first case,
There are total 8 electron and the electronic configuration is 1s2 2s2 2p4. The element which end up with 2p configuration belongs to 2nd period. It have four electron in their outermost shell so, it belongs to 16th group, So the element is Oxygen.
In the same way we will find the elements for rest of the cases.
Thus, we reach to the conclusion that the elements given the above orbital diagrams are as follows:
Oxygen
Oxygen
Aluminum
Nitrogen
learn more about stable electronic configuration here:
https://brainly.com/question/14275448
#SPJ10
Plowing is an example of what Energy?
a. Kinetic Energy
b. Potencial Energy
Answers
Answer:
mechanical or kinetic eg hammer and nails
If a wave of red light has a wavelength of 6.7 x 10-7 m, will the frequency of the red wave be high or low?
Answers
Answer:
Its high
Explanation:
Becuase if u times it what do u get